Product Name (品名): |
Valinomycin 缬氨霉素 |
Molecular Formula (分子式): |
C54H90N6O18
|
Molecular Weight (分子量): | 1111.32 |
| Production Capacity (产能) | 2000G/ Year |
Contact Telephone (联系电话): | 023-677770219 |
Contact | Sales Department |
| Contact Email: | sales@chemieliva.com |
S P E C I F I C A T I O N
|
| Appearance | White solid |
| Purity | ≥98%(TLC) & ≥95%(HPLC) |
| Melting Point | 187-190℃ |
| Specific Rotation | D20+31.0°(c=1.6 in benzene) |
Solubility | Soluble in DMSO at 10mg/ml, insoluble in water |
用途 Function Biological and Chemical Research Fields Tool for Potassium Ion Transport Mechanism Studies: It exhibits extremely high selective affinity for potassium ions (K⁺), forming lipophilic complexes to facilitate K⁺ transport across lipid bilayers. Commonly used to investigate the transport rules of intracellular and extracellular K⁺, it also helps construct pH environments required for acid-base jumps and K⁺-valinomycin diffusion potentials, aiding in the elucidation of ion transport mechanisms in biological membranes. Additionally, it can balance the intracellular and extracellular pH of strains such as Escherichia coli and lactic acid bacteria, providing conditions for related microbial metabolism research. Fabrication of Ion-Sensitive Devices: Using valinomycin as the active substance to prepare sensitive membranes, which are coated on the insulated gate surface of hydrogen ion-sensitive devices, enables the development of potassium ion-sensitive field-effect transistors (K⁺-sIFETs). These devices feature high stability, sensitivity, and selectivity, holding application value in the field of ion detection-related electronic devices. Chiral Separation Stationary Phase: As an optically active stationary phase, it is applicable in gas chromatography (GC), liquid chromatography (LC), and capillary electrophoresis (CE). Leveraging its unique molecular structure, it can achieve the resolution of chiral compounds, supporting the separation and detection of chiral substances in complex mixtures.
Biomedical Field Broad-Spectrum Antibacterial and Antiviral Activity: Initially isolated as an antibiotic, it exhibits antibacterial activity against Mycobacterium tuberculosis; it is also a highly effective antiviral agent, inhibiting various viruses including human coronaviruses, SARS-CoV, bunyaviruses, and enteroviruses, with potential value in antiviral drug research. Antitumor Research and Applications: As a mitophagy activator, it disrupts mitochondrial membrane potential and induces tumor cell apoptosis. When incorporated into liposomes, it reduces its own toxicity and enhances antitumor efficacy. In vitro experiments have shown significant inhibitory effects on various tumor cells such as hepatocellular carcinoma, ovarian cancer, and lung cancer, while in vivo experiments have demonstrated inhibition of tumor growth in nude mice.
Agricultural FieldValinomycin is an insecticidal antibiotic that can be isolated from the culture broth of strains such as Streptomyces griseus. It exhibits extremely strong inhibitory activity against mosquito larvae and can also be used to kill nematodes and waxworms. Furthermore, relevant studies have revealed its potential as a plant growth regulator, holding promise for further development and application in crop pest control and growth regulation.
|
Structure (结构式):
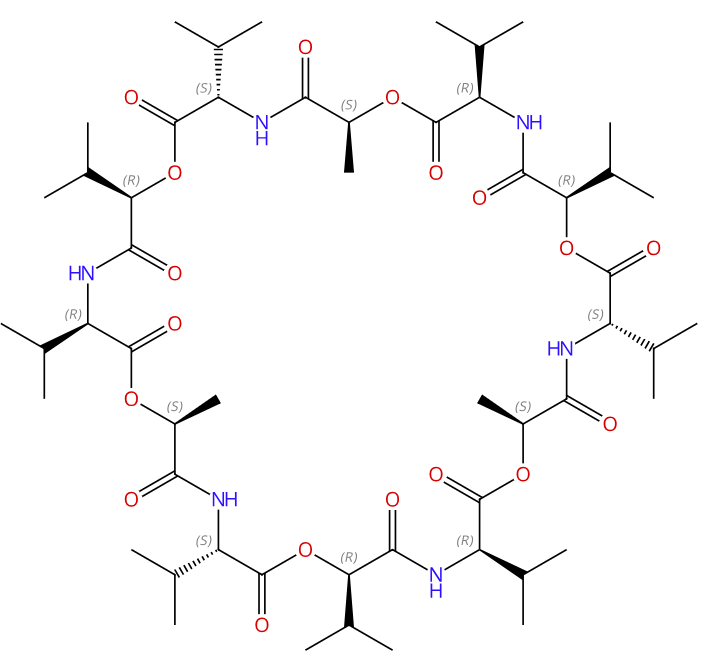
|
Valinomycin|| 缬氨霉素
2001-95-8 ||Chemieliva Pharmaceutical Co., Ltd || 重庆福腾医药|| 2001-95-8 seller || 2001-95-8 supplier || 2001-95-8 manufacturer || 2001-95-8 producer || 2001-95-8 price || 2001-95-8 China || 2001-95-8 India || 2001-95-8 MSDS || 2001-95-8 COA || CAS 2001-95-8 || buy 2001-95-8 || 2001-95-8 offer || 2001-95-8 seller || sell 2001-95-8 || selling 2001-95-8 || 2001-95-8 factory || 2001-95-8 factory || 2001-95-8 data || China 2001-95-8 || 2001-95-8 export ||
Canonical SMILES
O=C1OC(C(=O)NC(C(=O)OC(C(=O)NC(C(=O)OC(C(=O)NC(C(=O)OC(C(=O)NC(C(=O)OC(C(=O)NC(C(=O)OC(C(=O)NC1C(C)C)C(C)C)C(C)C)C)C(C)C)C(C)C)C(C)C)C)C(C)C)C(C)C)C(C)C)C
Isomeric SMILES
C(C)(C)[C@@H]1C(=O)N[C@H](C(C)C)C(=O)O[C@@H](C)C(=O)N[C@@H]([C@H](C)C)C(=O)O[C@H](C(C)C)C(=O)N[C@H](C(C)C)C(=O)O[C@@H](C)C(=O)N[C@@H]([C@@H](C)C)C(=O)O[C@H](C(C)C)C(=O)N[C@H](C(C)C)C(=O)O[C@@H](C)C(=O)N[C@@H]([C@H](C)C)C(=O)O1
InChI
InChI=1S/C54H90N6O18/c1-22(2)34-49(67)73-31(19)43(61)55-38(26(9)10)53(71)77-41(29(15)16)47(65)59-36(24(5)6)51(69)75-33(21)45(63)57-39(27(11)12)54(72)78-42(30(17)18)48(66)60-35(23(3)4)50(68)74-32(20)44(62)56-37(25(7)8)52(70)76-40(28(13)14)46(64)58-34/h22-42H,1-21H3,(H,55,61)(H,56,62)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t31-,32-,33-,34+,35+,36+,37-,38-,39-,40+,41+,42+/m0/s1
InChI Key
FCFNRCROJUBPLU-DNDCDFAISA-N
7 Other Names for this Substance
1,7,13,19,25,31-Hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane, cyclic peptide deriv. (ZCI)
1,7,13,19,25,31-Hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane-2,5,8,11,14,17,20,23,26,29,32,35-dodecone, 3,6,9,15,18,21,27,30,33-nonaisopropyl-12,24,36-trimethyl- (7CI)
Cyclo(D-α-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-α-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-α-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl)
MeSH ID: D014634
NSC 122023
Potassium ionophore I
Valinomicin










